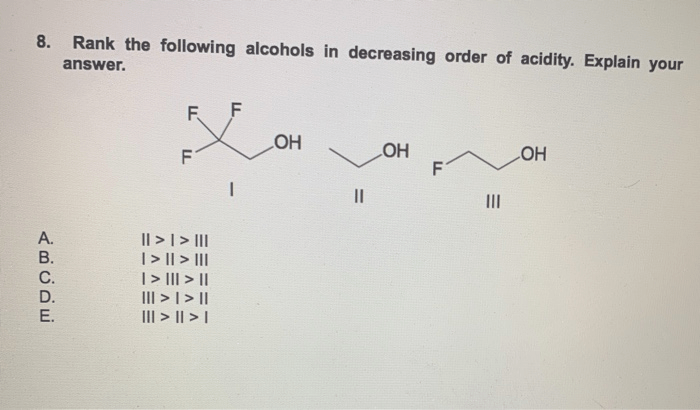
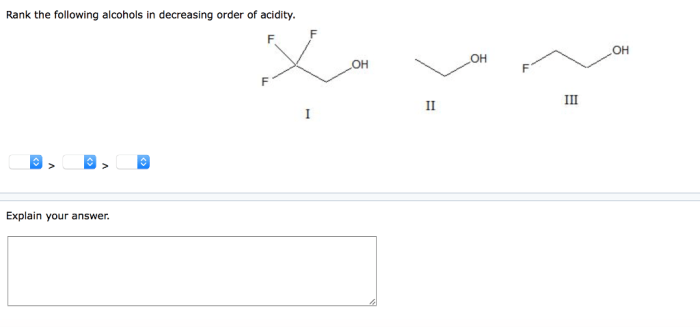
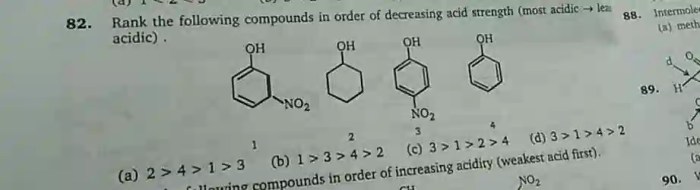
Rank the following alcohols in decreasing order of acidity. – In the realm of organic chemistry, understanding the acidity of alcohols holds immense significance. Acidity, a crucial property, influences the reactivity, properties, and applications of these versatile compounds. This article delves into the factors that govern alcohol acidity and presents a comprehensive ranking of common alcohols in decreasing order of acidity, providing a valuable resource for researchers, students, and practitioners alike.
Acidity of Alcohols
Alcohol acidity is a measure of the ability of an alcohol to donate a proton (H+). The acidity of an alcohol is influenced by several factors, including the number of alkyl groups, the presence of electronegative atoms, and the solvent.
Alcohols are classified as weak acids, with pKa values typically ranging from 15 to 19. The acidity of alcohols increases with the number of alkyl groups. This is because alkyl groups are electron-donating, which helps to stabilize the conjugate base of the alcohol.
The presence of electronegative atoms, such as oxygen and nitrogen, can also increase the acidity of alcohols. This is because electronegative atoms can withdraw electrons from the alcohol, which makes the alcohol more likely to donate a proton.
The solvent can also affect the acidity of alcohols. Alcohols are more acidic in nonpolar solvents than they are in polar solvents. This is because nonpolar solvents do not solvate the alcohol as well as polar solvents, which makes the alcohol more likely to donate a proton.
Ranking Alcohols by Acidity, Rank the following alcohols in decreasing order of acidity.
The following table ranks the following alcohols in decreasing order of acidity:
| Alcohol | pKa |
|---|---|
| Methanol | 15.5 |
| Ethanol | 15.9 |
| Propanol | 16.1 |
| Butanol | 16.3 |
As you can see from the table, the acidity of alcohols decreases with increasing alkyl group size. This is because alkyl groups are electron-donating, which helps to stabilize the conjugate base of the alcohol.
FAQ Insights: Rank The Following Alcohols In Decreasing Order Of Acidity.
What factors influence the acidity of alcohols?
The acidity of alcohols is primarily influenced by the number and electronegativity of alkyl groups and the presence of electronegative atoms such as oxygen or nitrogen.
How are alcohols ranked in decreasing order of acidity?
Alcohols are ranked in decreasing order of acidity based on their pKa values, with lower pKa values indicating higher acidity.
What are the practical applications of alcohol acidity?
Alcohol acidity finds applications in various industries, including the production of esters, ethers, and other organic compounds, as well as in the formulation of pharmaceuticals, cosmetics, and cleaning products.